Diffusion Restricted Contrast of Myocardium
- Zentralinstitut für Medizintechnik, Technische Universität München, Garching, Germany
The dependence of the measured diffusion tensor on diffusion time, in a diffusion tensor imaging (DTI) experiment has been reported for both the brain and the myocardium [1,2]. In the brain, it was shown that the rate of change of measured diffusion metrics with respect to diffusion times reveals novel contrast reflecting microstructure size [1] . The purpose of this study is to investigate if new tissue contrast in the myocardium can be obtained with DTI employing variable diffusion time.
DTI using diffusion encoded stimulated-echo acquisition mode sequence was performed on ex-vivo healthy pig hearts. Variable diffusion times were achieved by changing the distance between the second and third RF pulses. Diffusion times used in the experiment were 40 to 100 ms with steps of 10 ms [2] , [3]. Other imaging parameters were: b = 1000 s/mm2, 15 diffusion encoding directions, TE/TR = 59.6/3000 ms, 1 x 1 x 6 mm3 voxel size. A long axis slice through the left ventricle was acquired. The experiment was executed on a GE 3T MR750w scanner using a 16 element flex array coil fixed tightly around the heart. Linear least square fits of apparent diffusion coefficient (ADC) and eigenvalues (λ2, λ3) of the diffusion tensor with respect to diffusion times were performed on each voxel. Maps of resulted slopes of ADC, λ2, λ3 were generated.
Figure 1a shows the slope map of ADC relative to the diffusion time (rADC). Despite the existence of multiple fiber populations, as can be seen in the color-coded fractional anisotropy (cFA) map given in Figure 1b, the rADC map exhibits quite homogeneous contrast. Similar behavior was observed in the obtained slope maps for λ2, λ3 (data not shown). To have a closer look at the average slopes of different fiber populations, the cFA map (Figure 1b) was used to define three regions of interest (ROI) as shown. In the ROIs the mean and the standard deviation of rADC rλ2, rλ3 were calculated and given in Table 1. The fiber population in ROI 3 seems to have a slower rate of change in measured diffusion metrics, which possibly implies a less dense fiber concentration than in ROI 1 and ROI 2.
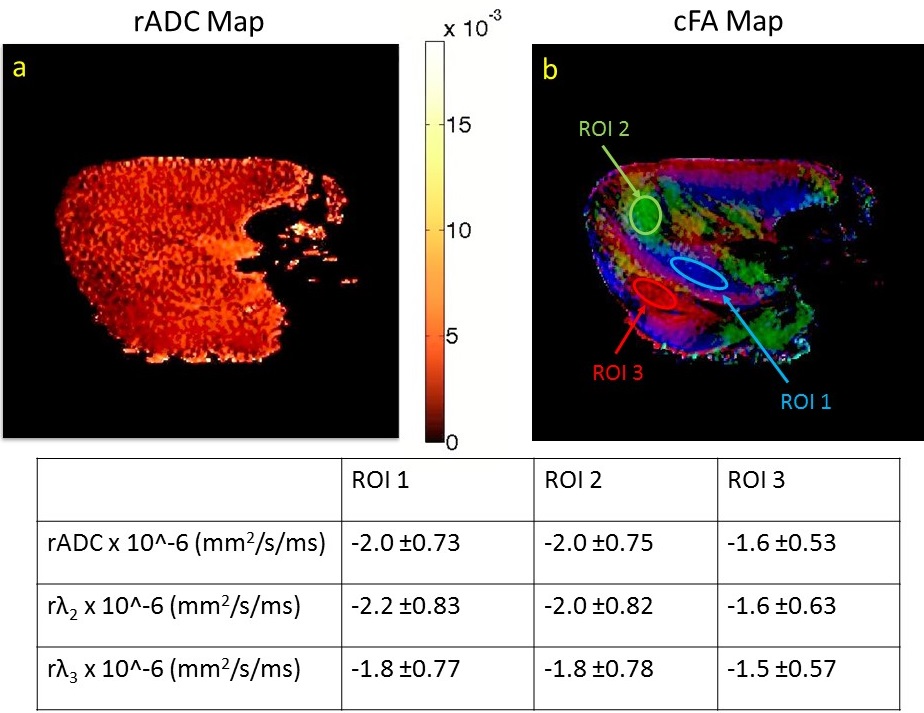
In conclusion, there is measurable difference in slopes of diffusion metrics with respect to diffusion times in different fiber population. We expect the difference to be more significant at shorter diffusion times, which is the next step of our study.
- [1] Aggarwal M, Jones MV, Calabresi PA, Mori S, Zhang J, (2012), Probing mouse brain microstructure using oscillating gradient diffusion MRI, Mag Reson Med, 98-109
- [2] S Kim, G Chi-Fishman, A S. Barnett, C Pierpaoli, (2005), Dependence on diffusion time of apparent diffusion tensor of ex vivio calf tongue and heart, Magn Reson Med, 1387-1396
- [3] Hoshino T, Fujuwara H, Kawai C, Hamashima Y, (1983), Myocardial fiber diameter and regional distributin in the ventricular wall of normal adult hearts, hypertensive hearts and hearts with hypertrophic cardiomyopathy, Circulation, 1109-1116
