Demonstration of In vivo Magnetic Resonance Imaging of Adult Zebrafish
- 1. University of Glasgow, Glasgow, United Kingdom
- 2. University of Edinburgh, Edinburgh, United Kingdom
Introduction
The zebrafish has seen increasing use as an research model, partly due to optically transparent nature of embryonic stage of the animal which allows for easy evaluation of internal anatomy and function. In contrast the adult form is optically opaque, necessitating the need for the development of alternative imaging modalities such as Magnetic Resonance Imaging (MRI). However, the small size and aquatic environment of the adult zebrafish has prevented the serious use of these alternatives. Scientific interest in adult phenotyping of zebrafish is rising. In vivo MRI of the adult zebrafish could therefore have significant scientific, ethical and economic benefits.
Methods
We developed an MRI scanning system to live adult zebrafish. This system consists of an integrated zebrafish life support/monitoring system (the flow cell) and a custom MRI solenoid microimaging coil (diameter 6.9mm) embedded within the flow cell (Fig. 1). Zebrafish were anesthetised (MS222) and held in position within the MRI coil. Water was flowed through the system via a non-pulsatile pump from outside the scanner room (2.00ml min-1). Temperature and oxygenation of water within the flow cell was monitored using in line MRI compatible optical sensors (FirestingO2, PyroScience). Scanning was performed on a 7T preclinical MRI scanner (Bruker BioSpec).
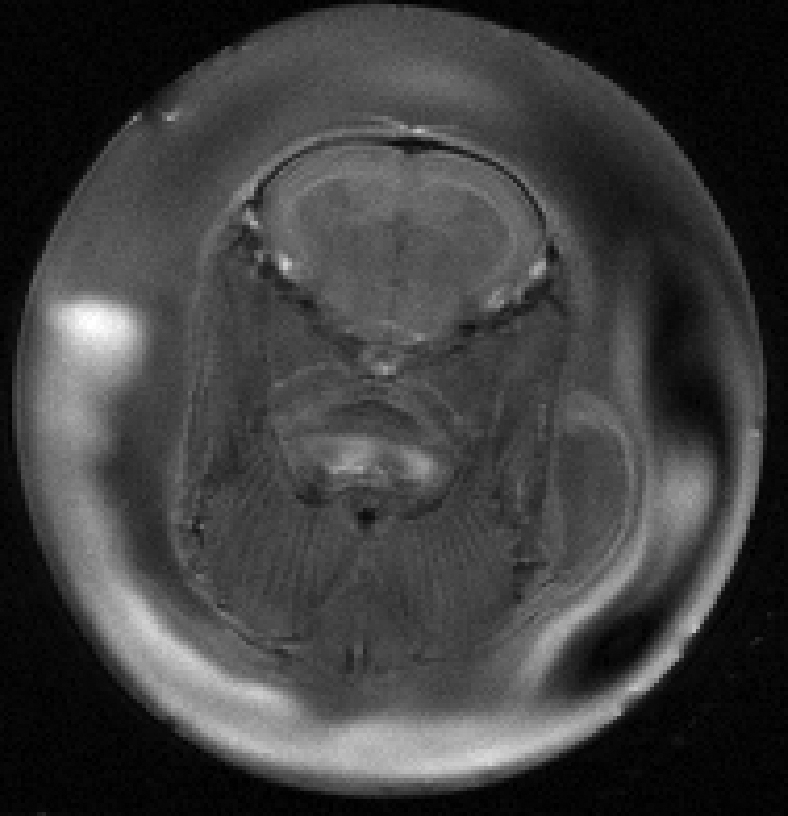
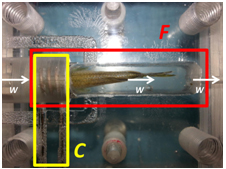
Results
We have created a working flow cell to obtain a range of common MRI scans in the live zebrafish. Zebrafish scanned under our current in vivo protocol were successfully recovered from the experimental procedure (up to 40mins) with no obvious signs of distress or injury allowing for longitudinal imaging studies.
Conclusion
We were able to successfully produce Zebrafish in vivo MRI images with high spatial resolution and image quality. Using our system non-invasive MRI studies of adult zebrafish are now possible.
Acknowledgments
This work is funded by a BHF New Horizons grant.
