MRI of Electroplating in Ionic Liquids
- 1. University of Birmingham, School of Chemistry, Birmingham, United Kingdom
- 2. University of Birmingham, School of Metallurgy and Materials, Birmingham, United Kingdom
- 3. University of Leicester, Chemistry Department, Leicester, United Kingdom
Recently developed techniques [1][2] for acquiring artifact-free MRI images near metallic electrodes have opened the door for in situ investigations of a wide range of applications in electrochemistry, among them the optimisation of electroplating using ionic liquid (IL) electrolytes. Electroplating is the electrochemical reduction of dissolved ions to produce metallic surface coatings, and ILs promise a greener alternative to the highly toxic aqueous processes that are currently used. For reactive metals such as aluminium, which cannot be electroplated in aqueous media, new IL-based processes also represent the only safe, affordable alternative, and thus may be key for a revolution in corrosion-resistant coatings. Development of these new techniques requires knowledge of the deposition mechanisms unique to ILs, which are typically inferred by post mortem analysis of the surfaces. However, relatively little is known about mechanisms of bulk transport and chemical speciation of the electroactive species during the plating process. These are critical factors in determining the quality of the coatings [3], and with the ability to spatially resolve electroplating cells non-destructively, MRI provides a powerful new tool to answer these questions and guide process optimisation in this burgeoning field.
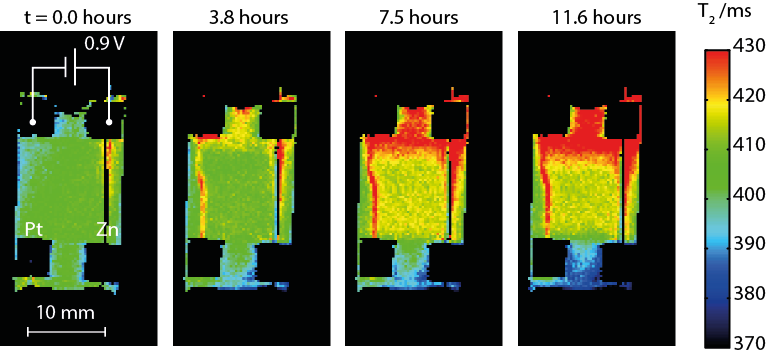
Experimentally, chemical sensitivity to the presence and concentration of the electroactive species is via the T1/T2 relaxation times of the solvent. ILs tend to co-ordinate with metal ions, leading to variation in relaxation times that provides a quantitative measure of concentration. Additionally, the anions and cations often contain distinct NMR-sensitive nuclei (1H or 19F, respectively), and the relaxation behaviour of each may yield clues about interactions between solvent and solute. Electroplating was performed in the magnet using a potentiostat with MR-compatible connections. By judicious cell design and alignment of the electrodes, artifact-free maps of relaxation times were acquired (Fig. 1), which reveal variations in composition of the IL as well as details of transport during dissolution of the anode and migration of the active species toward the cathode. In this paper, we present the first MRI results of zinc electroplating in ionic liquids and discuss what systems are amenable to study and how information on chemical species and transport correlates with traditional analysis of the electroplated surfaces.
- [1] Britton, M. M., et al. , (2013), In Situ, Real-Time Visualization of Electrochemistry Using Magnetic Resonance Imaging, The Journal of Physical Chemistry Letters, 4 (17): 3019-3023
- [2] Davenport, A. J., et al. , (2010), Visualisation of chemical processes during corrosion of zinc using magnetic resonance imaging, Electrochemistry Communications, 12(1): 44-47
- [3] Abbott, A. P., et al. , (2013), Electroplating Using Ionic Liquids, Annual Review of Materials Research, 43(1): 335-358
