Study of the Molecular Interactions of Ionic Liquid Colloidal Suspensions Using Rheometry and NMR
- University of Birmingham, School of Chemistry, Birmingham, United Kingdom
Colloidal suspensions are of significant interest in many areas, including structured materials (i.e. consumer products), solar cells[1] and nanoparticles synthesis.[2] Bulk rheological measurements are often used to investigate the dynamics and stability of these systems. For example, shear thinning behaviour is often associated with unstable suspensions containing aggregation of particles, whereas shear thickening can indicate a stabilised suspension.[3] Though such rheological phenomena have been widely observed in colloidal suspensions, the molecular origins of their non-Newtonian behaviour are still not fully understood.[4]
Imidazolium ionic liquids containing silica nanoparticles are ideal for solid state electrolytes.[5] Previous studies[3] of theses systems have found that the nature of the ionic liquid has a large effect of the stability, and hence the rheometry, of the suspension formed. However, the precise nature of the interactions between the silica nanoparticles and the ions of the ionic liquids is still unknown.
We have been studying the following range of imidazolium ionic liquids: [C2mim][BF4]; [C4mim][BF4]; [C2mim][NTf2] and [C4mim][NTf2]*. Bulk rheological measurements (figure 1a) have been combined with NMR velocimetry (figure 1b) to understand the local rheology of these complex fluids. Using multinuclear relaxation (figure 1c) and diffusion measurements we have been able to better understand the interactions between the silica nanoparticles with the cations and the anions of the ionic liquid which underpins the non-Newtonian rheology observed.
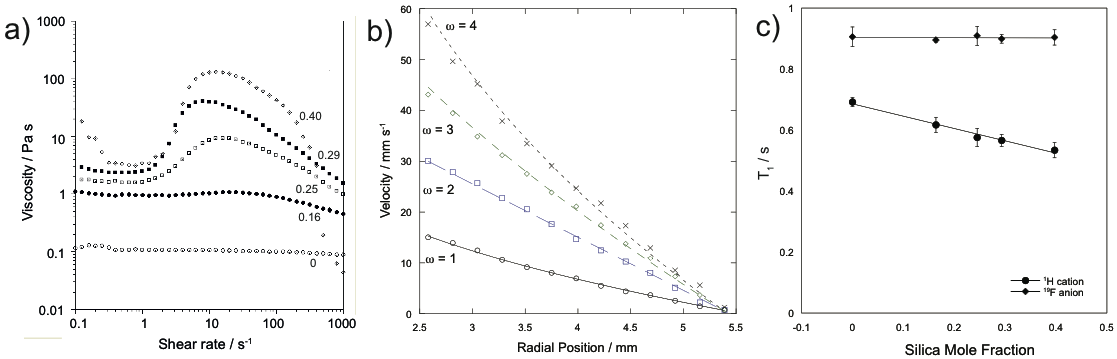
Figure 1: a) Plot of viscosity for [C4mim][BF4] with different mole fractions of silica; b) radial velocity profile for [C4mim][BF4] with 0.4 mole fraction of silica; c) 1H and 19F T1 relaxation times for [C4mim][BF4] with different mole fractions of silica.
NMR velocimetry has been used to observe the localised rheology of these systems in a Couette cell. By fitting the velocity profiles to the power law model[6], the power law exponent and hence the rheology of the fluids were identified. This data agrees well with conventional rheology measurements and suggests that the rheology of the ionic liquid is dependent on the anion; ionic liquids with the [BF4] anion showed shear thickening behaviour, while those with the [NTf2] anion showed shear thinning behaviour. In the case of the shear thickening system, [C4mim][BF4], (figure 1b) the power law exponent was found to increase with increasing shear rate. The pure ionic liquids showed Newtonian behaviour. These experiments suggest that the properties of the anion determine the rheology of the system.
The difference in rheology for the different anions have been associated with differences in the how these species interact with the silica nanoparticles.[3] However, there has been some disagreement in the literature over whether it is the anion or the cation of the ionic liquid interacting with the silica nanoparticles.[3][5][6] By using 1H NMR to observe the cation and 19F NMR to observe the anion, T1 measurements suggest that it is the cation of the ionic liquid that interacts with the silica nanoparticles (figure 1c). This has been observed in all four ionic liquid systems studied. Hence the molecular behaviour underpinning the rheology of these systems is more complex than previously thought. An explanation for the differing rheological behaviours of these systems will be presented in this talk.
*[C2mim]: 1-ethyl-3-methylimidazolium; [C4mim]: 1-butyl-3-methylimidazolium;
[BF4]: tetrafluoroborate; [NTf2]: bis(trifluoromethylsufonyl)imide
Colloidal suspensions are of significant interest in many areas, including structured materials (i.e. consumer products), solar cells[1] and nanoparticles synthesis.[2] Bulk rheological measurements are often used to investigate the dynamics and stability of these systems. For example, shear thinning behaviour is often associated with unstable suspensions containing aggregation of particles, whereas shear thickening can indicate a stabilised suspension.[3] Though such rheological phenomena have been widely observed in colloidal suspensions, the molecular origins of their non-Newtonian behaviour are still not fully understood.[4]
Imidazolium ionic liquids containing silica nanoparticles are ideal for solid state electrolytes.[5] Previous studies[3] of theses systems have found that the nature of the ionic liquid has a large effect of the stability, and hence the rheometry, of the suspension formed. However, the precise nature of the interactions between the silica nanoparticles and the ions of the ionic liquids is still unknown.
We have been studying[6] the following range of imidazolium ionic liquids: [C2mim][BF4]; [C4mim][BF4]; [C2mim][NTf2] and [C4mim][NTf2]*. Bulk rheological measurements (figure 1a) have been combined with NMR velocimetry (figure 1b) to understand the local rheology of these complex fluids. Using multinuclear relaxation (figure 1c) and diffusion measurements we have been able to better understand the interactions between the silica nanoparticles with the cations and the anions of the ionic liquid which underpins the non-Newtonian rheology observed.

Figure 1: a) Plot of viscosity for [C4mim][BF4] with different mole fractions of silica; b) radial velocity profile for [C4mim][BF4] with 0.4 mole fraction of silica; c) 1H and 19F T1 relaxation times for [C4mim][BF4] with different mole fractions of silica.
*[C2mim]: 1-ethyl-3-methylimidazolium; [C4mim]: 1-butyl-3-methylimidazolium;
[BF4]: tetrafluoroborate; [NTf2]: bis(trifluoromethylsufonyl)imide
- [1] A. Vioux, L. Viau, S. Volland and J. Le Bideau, (2010), Use of ionic liquids in sol-gel; ionogels and applications, C. R. Chemie, 242, 13
- [2] A. Wittmar, D. Ruiz-Abad and M. Ulbricht, (2012), Dispersions of silica nanoparticles in ionic liquids investigated with advanced rheology, J Nanopart. Res., 651, 14
- [3] K. Ueno and M. Watanabe, (2011), From Colloidal Stability in Ionic Liquids to Advanced Soft Materials Using Unique Media, Langmuir, 9105, 11
- [4] X. Cheng, J. H. McCoy, J. N. Israelachvili and I. Cohen, (2011), Imaging the Microscopic Structure of Shear Thinning and Thickening Colloidal Suspensions, Science, 1276, 333
- [5] S. Shimano, H. Zhou and I. Honma, (2007), Preparation of Nanohybrid Solid-State Electrolytes with Liquidlike Mobilities by Solidifying Ionic Liquids with Silica Particles, Chem Mater, 5216, 19
- [6] J. Novak and M. M. Britton, (2013), Magnetic resonance imaging of the rheology of ionic liquid colloidal suspensions, Soft Matter, 2730, 9
