The urea transporter - an MRI gene reporter that can be detected using transmembrane water exchange imaging
- 1. University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, United Kingdom
- 2. Technische Universität München, Nuklearmedizinische Klinik und Poliklinik, München, Germany
AIMS
Monitoring gene expression in vivo using non-invasive molecular imaging techniques could be used in the clinic to monitor gene therapy and for cell tracking in regenerative medicine. In the case of MRI, various reporter genes, with different underlying contrast mechanisms and substrates, have been developed (T1,T2,T2*,chemical exchange saturation transfer (CEST), apparent diffusion coefficient (ADC)).[1][2] However, many of these approaches suffer from low sensitivity and would be difficult to translate into clinical use. Recently a urea transporter (UTB) has been described as an MRI gene reporter, where reporter expression was detected in vivo using 13C MRS ADC measurements following injection of hyperpolarized 13C urea.[3] UTBs also transport water, at similar rates to aquaporin channels[4], and we show here that UTB transporter expression can also be detected without the use of exogenous substrates by using 1H MRI measurements of transmembrane water exchange.
METHODS
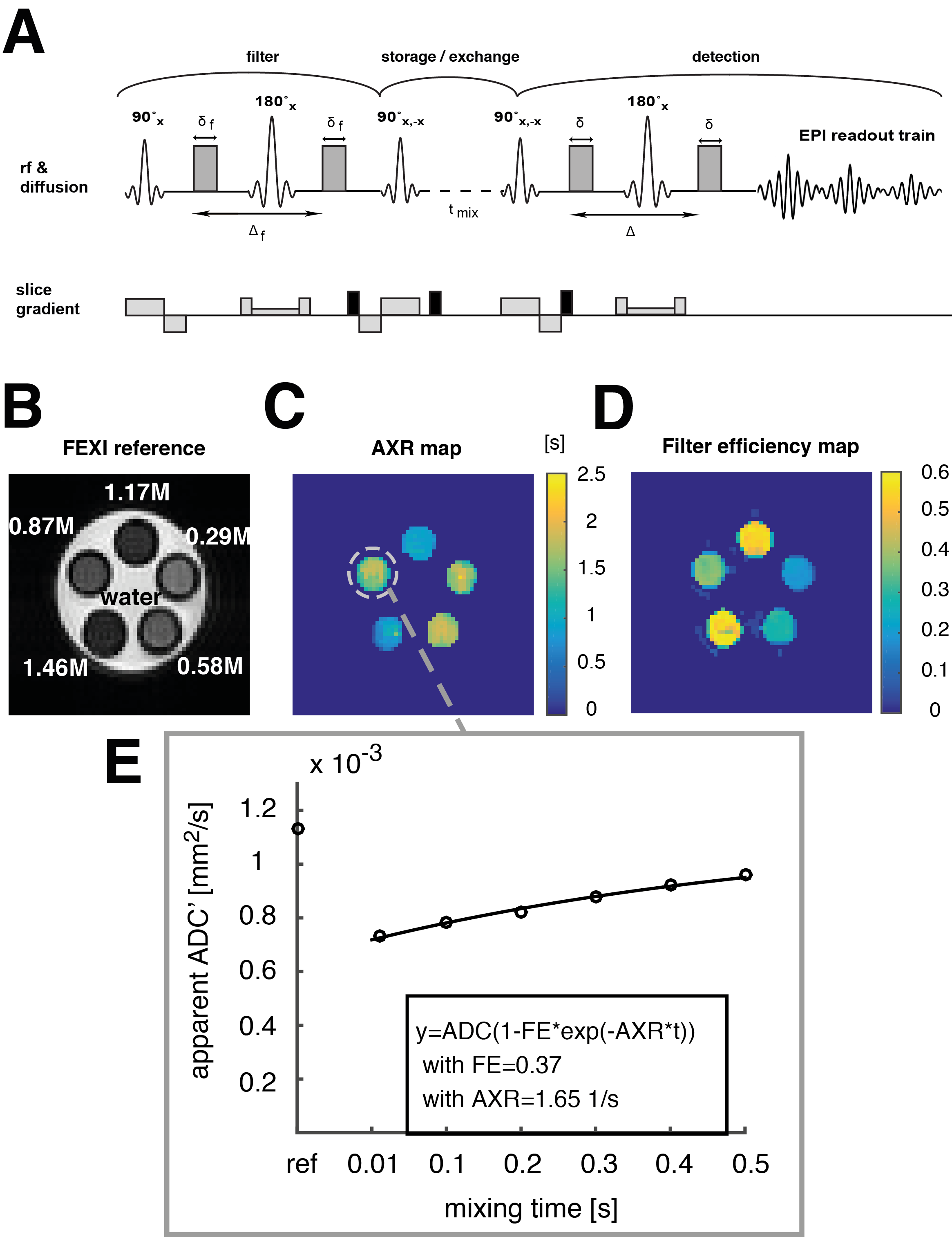
An exchange-sensitive filter-exchange imaging (FEXI)[5][6] sequence was implemented and optimized on a 9.4 T animal scanner using a phantom containing different compartments with sucrose concentrations in the range from 0.3M to 1.5M (Fig. 1 A,B). The storage and recovery pulses (pulse 3 and 4) were phase cycled to lead to a first order correction of T1 influences during the storage module. Spoiler gradients were employed before, during, and after the storage module to remove unwanted coherence pathways. Sucrose has exchangeable hydroxyl-protons and a decrease in water mobility is observed at high sugar concentrations due to hydrogen bonding between water and sucrose molecules and between the sucrose molecules themselves.
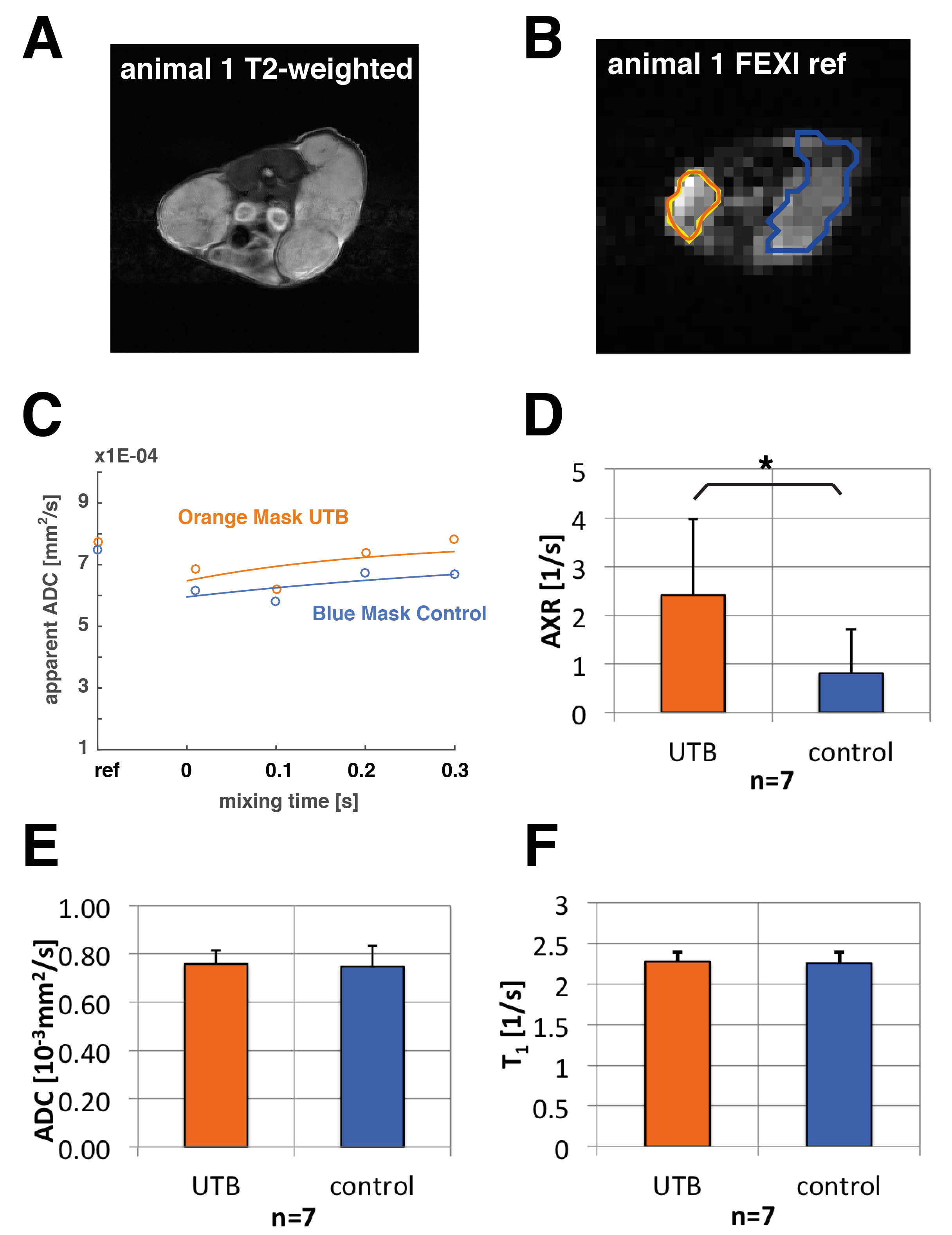
HEK 293T cells that had been transduced with a UTB-expressing lentiviral vector were grown in DMEM. Eight immune-deficient mice were implanted subcutaneously with 1x107 UTB-expressing cells on one flank and unmodified cells on the contralateral flank. Animals were imaged using FEXI for transmembrane water exchange measurements, Inversion Recovery Turbo-FLASH for T1 mapping, and diffusion-weighted spin echo EPI for ADC measurements. In vivo FEXI parameters were: diffusion filter b-values (bf=(0;2183)s/mm2; detection b-values b=(36;517)s/mm2; mixing times (acquired interleaved) tmix=(0.01;0.1;0.2;0.3)s; Δ =0.012ms; δ=0.08ms; matrix:32x32; slice thickness: 3mm; FOV: 4cm x 4cm; the sequence was gated with a respiration sensor leading to a TR of ca. 3s; total acquisition time was ca. 15min.
RESULTS & DISCUSSION
The apparent exchange rates of water protons in sucrose solutions at hydroxyl-proton exchange rates were measured and imaged in the NMR slow-exchange regime at sucrose concentrations above 0.5M (Fig. 1 C,E). A reduction in the apparent proton exchange rate (AXR) with increasing sucrose concentrations was revealed, suggesting that hydrogen bonding between sucrose molecules, by competing with hydrogen bonding between sucrose and water, slows the water proton exchange.
In vivo FEXI datasets were evaluated in 7 out of 8 animals (one of them was excluded because of severe motion artefacts) to fit apparent exchange rates (AXRs) in xenograft ROIs (Fig. 2). AXR values in the UTB-expressing xenografts were significantly higher (p ≤0.05, unpaired two-tailed Student's t-test) with a mean AXR of (2.41±1.56)1/s for UTB and (0.81±0.89)1/s for the control xenografts (Fig. 2 D). All errors are indicated as standard deviations. T1 and ADC maps for the same ROIs did not show significant differences between UTB and control tumours (Fig. 2 E, F). Voxel-based calculation of AXR maps was also done in the animals (data not shown) and shows promise for tracking UTB-transfected cells in vivo. Further improvements in SNR and AXR mapping are expected by increasing the number of averages and reducing the number of mixing times to a 2-point measurement, as well as better fixation of the animals to reduce motion artefacts.
CONCLUSION
Genetically induced manipulation of transmembrane water exchange through expression of urea transporters (UTB) can be quantified by filter-exchange imaging techniques. Building on endogenous water as a substrate and clinically established diffusion imaging techniques, UTB shows promise for translation as a clinical MRI gene reporter.
-
- [1] R. Weissleder, A. Moore, U. Mahmood, R. Bhorade, H. Benveniste, E.A. Chiocca, J.P. Basilion, (2000), In vivo magnetic resonance imaging of transgene expression, Nature Medicine 6, 351
- [2] M.H. Vandsburger, M. Radoul, B. Cohen, M. Neeman, (2013), MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation, NMR in Biomedicine 26, 872
- [3] B. Yang, A.S. Verkman, (2002), Analysis of Double Knockout Mice Lacking Aquaporin-1 and Urea Transporter UT-B, The Journal of Biological Chemistry 277
- [4] P.S. Patrick, M.I. Kettunen, S.-S. Tee, T.B. Rodrigues, E. Serrao, K.N. Timm, S. McGuire, K.M. Brindle , (2015), Detection of Transgene Expression Using Hyperpolarized 13C Urea and Diffusion-Weighted Magnetic Resonance Spectroscopy, Magnetic Resonance in Medicine 73, 1401
- [5] S. Lasic, M. Nilsson, J. Lätt, F. Stahlberg, D. Topgaard, (2011), Apparent Exchange Rate Mapping with Diffusion MRI, Magnetic Resonance in Medicine 66, 356
- [6] M. Nilsson, J. Lätt, D. van Westen, S. Brockstedt, S. Lasic, F. Stahlberg, D. Topgaard, (2013), Noninvasive Mapping of Water Diffusional Exchange in the Human Brain Using Filter-Exchange Imaging, Magnetic Resonance in Medicine 69, 1573
