Unique nature of ion induced phase transition in gels revealed using NMR
- 1. Tel-Aviv University, Biomedical Engineering, Tel-Aviv, Israel
- 2. Tel-Aviv University, Chemistry, Tel-Aviv, Israel
- 3. Tel-Aviv University, Sagol School of Neuroscience, Tel-Aviv, Israel
Volume phase transition is a universal phenomenon that was observed in many gels, both synthetic and natural. This behavior is evident in systems where smooth change of environmental parameters result in a sharp change in the gel volume. Understanding the gel along a phase transition may be of great interest to both: (a) Biophysical studies, where previous observations of diffusion changes in nerve cells was suggested to be related to a ion induced volume change of the biological gel in the cell [3]; (b) Practical applications development, in which gels can be a key components to systems such as drug release, enzyme control, sensors, and actuators and artificial muscles.
This work was conducted using a synthetic poly(acrylic-acid) as a model. It presents a unique interpretation of standard NMR measurements (R2 presented in Fig. 1(a) and Triple Quantum Filtering (TQF) of 1H and 23Na. The results were analyzed by applying the BPP theory [5] and a simple two-population model of bound and free water, Figs. 1(b) and 1(c). This enables the prediction of bound water fraction (the polymer hydration layer) and correlation time of both populations.
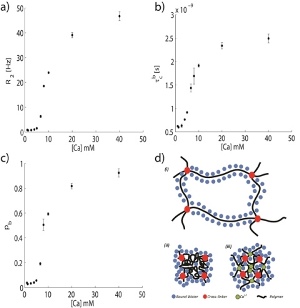
Significant conclusions were: (1) Correlation time of free ('Bulk') water in the gel is similar to that of pure water, even in compact gels. Correlation time of bound water is 3 orders of magnitude higher, indicating two distinguishable populations. Similar results are found for 23Na; (2) Ion induced and temperature induced phase transitions are different in the contribution of bound water to the gel's shrinkage. In ion induced phase transition only free water exit the gel, and the amount of bound water remains constant (see Fig. 1(d)). (3) R1 and R2 values are mostly sensitive to bound population dynamics. The ADC value, on the other hand, almost solely depends on free molecules dynamic.
- [1] Moseley, M. E. et al., (1990), Early Detection of Regional Cerebral-Ischemia in Cats - Comparison of Diffusion-Weighted and T2-Weighted Mri and Spectroscopy, Magnetic Resonance in Medicine, 330-346
- [2] Tirosh, N. and Nevo, U., (2013), Neuronal activity significantly reduces water displacement: DWI of a vital rat spinal cord with no hemodynamic effect, Neuroimage, 98-107
- [3] Tasaki, I., (1982), Physiology and electrochemistry of nerve fibers, Academic Press, New York, 256-280
- [4] Hubbard, P. S., (1970), Nonexponential Nuclear Magnetic Relaxation by Quadrupole Interactions, Journal of Chemical Physics, 985
- [5] Abragam, A., (1961), The Principles of Nuclear Magnetism, Clarendon Press
- [6] Jaccard, J. et al., (1986), Multiple quantum NMR spectroscopy of S= 3/2 spins in isotropic phase: A new probe for multiexponential relaxation, The Journal of chemical physics, 6282-6293
